Patient and Public Involvement in Research
Understanding health and social care research is essential. It’s not just about lab experiments; it’s about improving health and well-being for everyone. As a member of the public, your insights and experiences play a crucial part. Imagine a jigsaw puzzle: researchers have some pieces (clinical studies), but they need your unique ones – your real-world stories and perspectives. Patients, carers, and members of the public like you contribute to the bigger picture. Together, we shape better care for future generations. If you’re curious about getting involved, explore the information below to learn more. Let’s make a meaningful impact together!
Remember you can be an active partner in advancing health knowledge!
What Is Patient and Public Involvement (PPI) in Research?
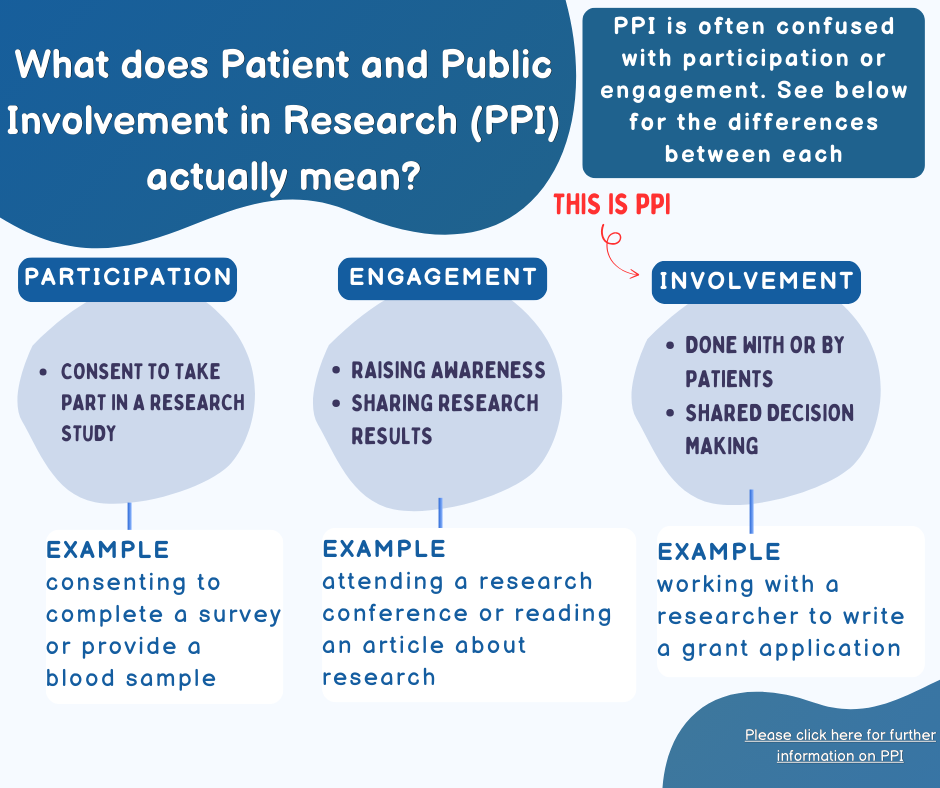
- In research, PPI means involving regular people (like patients and the public) in planning and doing research. They give their opinions and ideas to make sure the research is helpful and fair
- It’s about collaboration, not just participation. For example, patients and the public contribute to research design, conduct, and reporting the findings
- Think of it as making sure everyone’s voice is heard. When researchers work with patients and the public, they get better ideas and make sure the research is useful for real-life situations
- PPI does not refer to research participants taking part in a study
How are Patients and the Public involved in Research at the CRF?
The CRF aims to be a champion of patient involvement in research and we endeavour to include patients and the public in research activity wherever possible. Below you will find some examples of our PPI work.
- The CRF Board of Governance
- The operational managers of the CRF report to this Board who provide strategic direction and oversight of CRF activity. A patient representative sits on the Board.
- CRF Grant Oversight Committee
- The CRF hosts an oversight committee to provide guidance and feedback on knowledge translation activities. This committee has two patient representatives.
- Reporting and Talking about Research
- The CRF has recently begun working with the hospital Intensive Care Unit Education Team to produce a Research Podcast Miniseries. Patient representative groups at the hospital have been included in the design of this project. We are particularly proud to say that patient representatives also co-host the podcasts. You can listen to them here.
- During Science Week Ireland (2022), ‘Hormones Explained for Teens’ for RTE Kids, was co-written and presented by Dr Roshaida Abdul Wahab who has conducted research at the CRF.
How can I get involved?
Please contact the CRF by Phone on +353 1 4103900 to express your interest in becoming involved in research.