Our Impact
Since opening in May 2013, the CRF has grown from strength to strength supporting a wide variety of clinical trials and studies Exciting milestones at the CRF includes:
- The first gene therapy clinical trial in Ireland for patients with haemophilia
- Multiple Phase 1 overnight clinical trials involving an incurable neurological condition
Disease Areas
19
Investigators
140
Regulated Clinical Trials
88
Clinical Studies
143
Our Strengths & Expertise
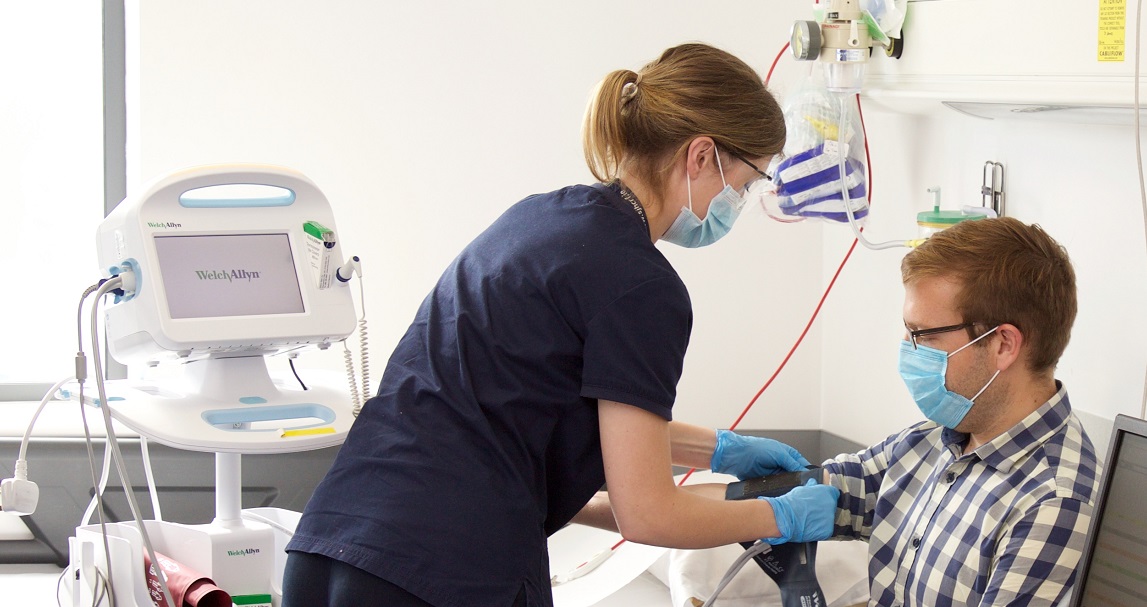
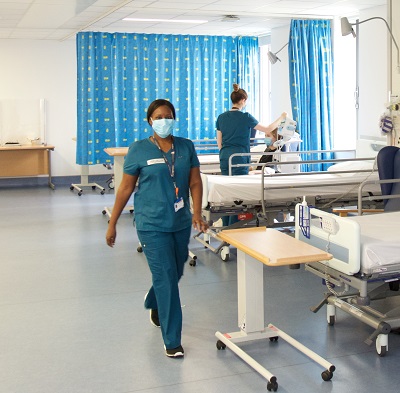
Located in the heart of St James’s hospital, we deliver on innovative, complex clinical trials supporting Investigators and their patients every step of the way.